Viona Pharma Recalls Metformin HCl Extended- Release Tablets
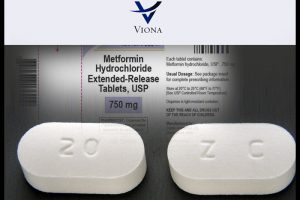
Viona Pharmaceuticals Inc. is recalling twenty-three lots of type 2 diabetes drug Metformin Hydrochloride or HCI Extended-Release Tablets, USP 750 mg at the consumer level due to the detection of N-Nitrosodimethylamine or NDMA impurity, the U.S. Food and Drug Administration said.
The tablets are used as an adjunct to diet and exercise to improve blood glucose control in adults with type 2 diabetes mellitus.
NDMA is classified as a probable human carcinogen, which is a substance that could cause cancer, based on results from laboratory tests. NDMA is a known environmental contaminant and found in water and foods, including meats, dairy products, and vegetables.
According to the agency, the recall was initiated after an Out of Specification or OOS result observed for one lot of the product (M008132).
However, in an abundance of caution, Cranford, New Jersey-based company has decided to recall 23 batches which is determined to have a valid shelf life within the US market.
The recalled tablets are packaged in HDPE bottles of 100 tablets, under NDC 72578-036-01. The product was manufactured by Cadila Healthcare Limited, Ahmedabad, India for U.S. distribution by Viona Pharma, and was sold across the United States.
According to the FDA, it could be dangerous for patients with type 2 diabetes to stop taking their Metformin without first talking to their healthcare professionals. Patients who have received impacted lots of Metformin are advised to continue taking their medication and contact their physician for advice regarding an alternative treatment.
However, Viona Pharma and Cadila Healthcare have not received any reports of adverse events related to the recall to date.
Viona Pharma in late December recalled Metformin in the form of oral film-coated tablets for the potential presence of NDMA above levels of the Acceptable Daily Intake Limit. In June last year, the company had called back 2 lots of Metformin HCl Extended Release tablets USP 750 mg for similar issues.
Source: Read Full Article
